Nápady 89 Atom Economy Formula Gcse
Nápady 89 Atom Economy Formula Gcse. The atom economy could also be calculated using mass, instead or mr. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100
Prezentováno Percentage Yield And Atom Economy Science And Joe
(𝑅 𝑎 𝑖 𝑎 𝑎 𝑖 𝑎 𝑖 ) 𝑥 100 (1 mark) using concentrations of solutions in mol/dm3 The percentage atom economy of a reaction is calculated using this equation: Yield & atom economy | aqa gcse chemistry | questions & answers. The reaction is as follows: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2.Ch 4+ 2h 2o→co 2+ 4h 2 formula mass:
Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. % atom economy = (4 / 36) * 100 = 11.1%. Download all our revision notes as pdfs. The percentage atom economy of a reaction is calculated using this equation: In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. However, with plenty of revision, memorising of the formula's becomes easier, understanding atom economy becomes second nature and applying avagadro's constant will become straight forward.

Download all our revision notes as pdfs. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. The atom economy could also be calculated using mass, instead or mr. From understanding avagadro's contact, to mole calculations, formula's for percentage yield and atom economy, at first this part of the gcse chemistry syllabus seems very difficult. Download all our revision notes as pdfs. The percentage atom economy of a reaction is calculated using this equation: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. % atom economy = (4 / 36) * 100 = 11.1%. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: Calculating atom economy atom economy can be calculated using this equation: However, with plenty of revision, memorising of the formula's becomes easier, understanding atom economy becomes second nature and applying avagadro's constant will become straight forward. % atom economy = (4 / 36) * 100 = 11.1%.

However, with plenty of revision, memorising of the formula's becomes easier, understanding atom economy becomes second nature and applying avagadro's constant will become straight forward. The atom economy could also be calculated using mass, instead or mr. The reaction is as follows: Use our chemistry revision … Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: Download all our revision notes as pdfs. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. Complete the equation for how atom economy is calculated. Yield & atom economy | aqa gcse chemistry | questions & answers.. However, with plenty of revision, memorising of the formula's becomes easier, understanding atom economy becomes second nature and applying avagadro's constant will become straight forward.
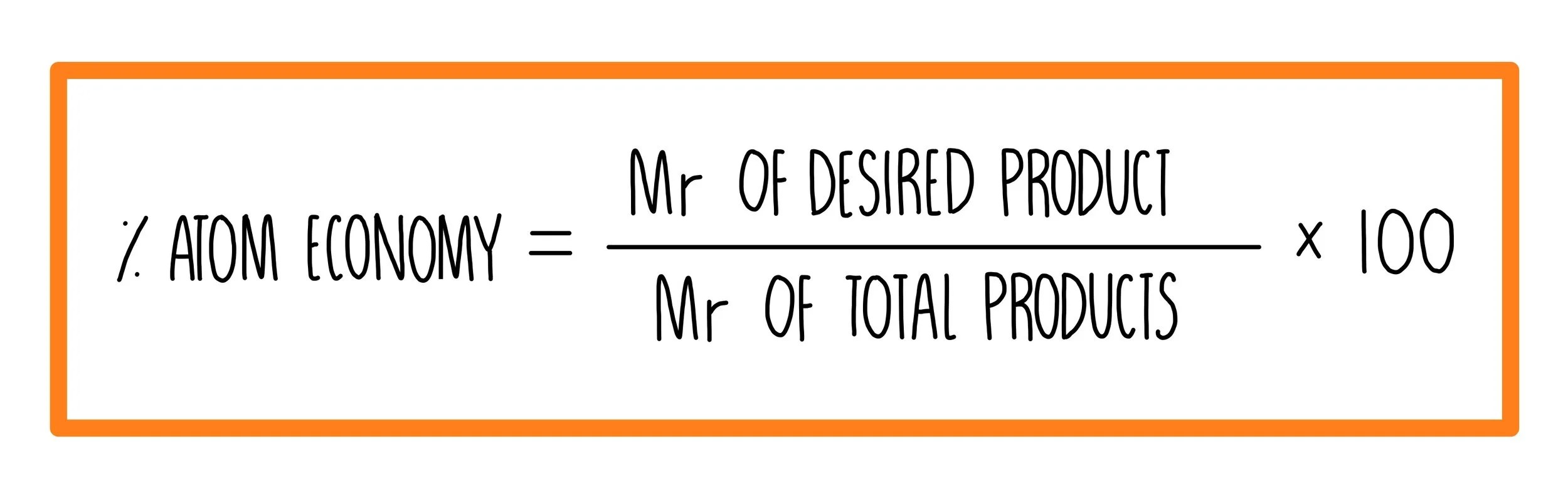
The percentage atom economy of a reaction is calculated using this equation:. Download all our revision notes as pdfs. Calculating atom economy atom economy can be calculated using this equation: The atom economy could also be calculated using mass, instead or mr. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Between the steam reforming reaction and the. The percentage atom economy of a reaction is calculated using this equation: (𝑅 𝑎 𝑖 𝑎 𝑎 𝑖 𝑎 𝑖 ) 𝑥 100 (1 mark) using concentrations of solutions in mol/dm3 The percentage atom economy of a reaction is calculated using this equation: Ch4 (g) + h2o (g) → co (g) + … However, with plenty of revision, memorising of the formula's becomes easier, understanding atom economy becomes second nature and applying avagadro's constant will become straight forward... Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2.

However, with plenty of revision, memorising of the formula's becomes easier, understanding atom economy becomes second nature and applying avagadro's constant will become straight forward.. Between the steam reforming reaction and the. The atom economy could also be calculated using mass, instead or mr. Complete the equation for how atom economy is calculated.. % atom economy = (4 / 36) * 100 = 11.1%.

Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Complete the equation for how atom economy is calculated. Complete the equation for how atom economy is calculated.

Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100 The percentage atom economy of a reaction is calculated using this equation: Ch4 (g) + h2o (g) → co (g) + … The atom economy could also be calculated using mass, instead or mr. Yield & atom economy | aqa gcse chemistry | questions & answers. (𝑅 𝑎 𝑖 𝑎 𝑎 𝑖 𝑎 𝑖 ) 𝑥 100 (1 mark) using concentrations of solutions in mol/dm3 Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100 Then, we calculate % atom economy: Complete the equation for how atom economy is calculated. Between the steam reforming reaction and the.. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g.

Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Use our chemistry revision … The percentage atom economy of a reaction is calculated using this equation: Calculating atom economy atom economy can be calculated using this equation: Complete the equation for how atom economy is calculated. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: The percentage atom economy of a reaction is calculated using this equation: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. However, with plenty of revision, memorising of the formula's becomes easier, understanding atom economy becomes second nature and applying avagadro's constant will become straight forward. Download all our revision notes as pdfs. Yield & atom economy | aqa gcse chemistry | questions & answers.

Then, we calculate % atom economy: Download all our revision notes as pdfs. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. The atom economy could also be calculated using mass, instead or mr.. The reaction is as follows:

The percentage atom economy of a reaction is calculated using this equation: The percentage atom economy of a reaction is calculated using this equation: Ch4 (g) + h2o (g) → co (g) + … Then, we calculate % atom economy: (𝑅 𝑎 𝑖 𝑎 𝑎 𝑖 𝑎 𝑖 ) 𝑥 100 (1 mark) using concentrations of solutions in mol/dm3 Between the steam reforming reaction and the. Complete the equation for how atom economy is calculated.. However, with plenty of revision, memorising of the formula's becomes easier, understanding atom economy becomes second nature and applying avagadro's constant will become straight forward.

Then, we calculate % atom economy: Ch4 (g) + h2o (g) → co (g) + … From understanding avagadro's contact, to mole calculations, formula's for percentage yield and atom economy, at first this part of the gcse chemistry syllabus seems very difficult. However, with plenty of revision, memorising of the formula's becomes easier, understanding atom economy becomes second nature and applying avagadro's constant will become straight forward.
08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. Download all our revision notes as pdfs. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Then, we calculate % atom economy: Complete the equation for how atom economy is calculated. Yield & atom economy | aqa gcse chemistry | questions & answers. Use our chemistry revision … % atom economy = (4 / 36) * 100 = 11.1%. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. Calculating atom economy atom economy can be calculated using this equation: Between the steam reforming reaction and the.. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy.

Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100. (𝑅 𝑎 𝑖 𝑎 𝑎 𝑖 𝑎 𝑖 ) 𝑥 100 (1 mark) using concentrations of solutions in mol/dm3 Complete the equation for how atom economy is calculated.. Ch4 (g) + h2o (g) → co (g) + …

However, with plenty of revision, memorising of the formula's becomes easier, understanding atom economy becomes second nature and applying avagadro's constant will become straight forward. % atom economy = (4 / 36) * 100 = 11.1%. From understanding avagadro's contact, to mole calculations, formula's for percentage yield and atom economy, at first this part of the gcse chemistry syllabus seems very difficult. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Calculating atom economy atom economy can be calculated using this equation: Use our chemistry revision …

(𝑅 𝑎 𝑖 𝑎 𝑎 𝑖 𝑎 𝑖 ) 𝑥 100 (1 mark) using concentrations of solutions in mol/dm3.. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. Between the steam reforming reaction and the. However, with plenty of revision, memorising of the formula's becomes easier, understanding atom economy becomes second nature and applying avagadro's constant will become straight forward. Download all our revision notes as pdfs. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100.

Yield & atom economy | aqa gcse chemistry | questions & answers. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Between the steam reforming reaction and the. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. The percentage atom economy of a reaction is calculated using this equation: Complete the equation for how atom economy is calculated. Then, we calculate % atom economy: 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. Yield & atom economy | aqa gcse chemistry | questions & answers. The atom economy could also be calculated using mass, instead or mr. Ch4 (g) + h2o (g) → co (g) + ….. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass:

Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. The reaction is as follows: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. Then, we calculate % atom economy: The percentage atom economy of a reaction is calculated using this equation: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. (𝑅 𝑎 𝑖 𝑎 𝑎 𝑖 𝑎 𝑖 ) 𝑥 100 (1 mark) using concentrations of solutions in mol/dm3. However, with plenty of revision, memorising of the formula's becomes easier, understanding atom economy becomes second nature and applying avagadro's constant will become straight forward.

Ch4 (g) + h2o (g) → co (g) + … Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: Then, we calculate % atom economy: In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. Ch4 (g) + h2o (g) → co (g) + … % atom economy = (4 / 36) * 100 = 11.1%. Download all our revision notes as pdfs. Between the steam reforming reaction and the. Complete the equation for how atom economy is calculated. Yield & atom economy | aqa gcse chemistry | questions & answers. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass:

Between the steam reforming reaction and the.. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. However, with plenty of revision, memorising of the formula's becomes easier, understanding atom economy becomes second nature and applying avagadro's constant will become straight forward. (𝑅 𝑎 𝑖 𝑎 𝑎 𝑖 𝑎 𝑖 ) 𝑥 100 (1 mark) using concentrations of solutions in mol/dm3 Ch4 (g) + h2o (g) → co (g) + … Then, we calculate % atom economy: Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100. Calculating atom economy atom economy can be calculated using this equation:

The percentage atom economy of a reaction is calculated using this equation: Between the steam reforming reaction and the. However, with plenty of revision, memorising of the formula's becomes easier, understanding atom economy becomes second nature and applying avagadro's constant will become straight forward. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. Use our chemistry revision … Download all our revision notes as pdfs. From understanding avagadro's contact, to mole calculations, formula's for percentage yield and atom economy, at first this part of the gcse chemistry syllabus seems very difficult. (𝑅 𝑎 𝑖 𝑎 𝑎 𝑖 𝑎 𝑖 ) 𝑥 100 (1 mark) using concentrations of solutions in mol/dm3 Calculating atom economy atom economy can be calculated using this equation: Then, we calculate % atom economy: The atom economy could also be calculated using mass, instead or mr. (𝑅 𝑎 𝑖 𝑎 𝑎 𝑖 𝑎 𝑖 ) 𝑥 100 (1 mark) using concentrations of solutions in mol/dm3

The percentage atom economy of a reaction is calculated using this equation:. Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100 Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Ch4 (g) + h2o (g) → co (g) + … The percentage atom economy of a reaction is calculated using this equation: Download all our revision notes as pdfs. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Use our chemistry revision … Between the steam reforming reaction and the. Yield & atom economy | aqa gcse chemistry | questions & answers.

Yield & atom economy | aqa gcse chemistry | questions & answers... Download all our revision notes as pdfs. Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100 Then, we calculate % atom economy: (𝑅 𝑎 𝑖 𝑎 𝑎 𝑖 𝑎 𝑖 ) 𝑥 100 (1 mark) using concentrations of solutions in mol/dm3 Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. The reaction is as follows: % atom economy = (4 / 36) * 100 = 11.1%.

Download all our revision notes as pdfs. Calculating atom economy atom economy can be calculated using this equation: Between the steam reforming reaction and the. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Yield & atom economy | aqa gcse chemistry | questions & answers. Download all our revision notes as pdfs. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. The reaction is as follows: However, with plenty of revision, memorising of the formula's becomes easier, understanding atom economy becomes second nature and applying avagadro's constant will become straight forward. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100. Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100

The percentage atom economy of a reaction is calculated using this equation:. Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100 (𝑅 𝑎 𝑖 𝑎 𝑎 𝑖 𝑎 𝑖 ) 𝑥 100 (1 mark) using concentrations of solutions in mol/dm3 % atom economy = (4 / 36) * 100 = 11.1%. However, with plenty of revision, memorising of the formula's becomes easier, understanding atom economy becomes second nature and applying avagadro's constant will become straight forward. Then, we calculate % atom economy: The atom economy could also be calculated using mass, instead or mr. The percentage atom economy of a reaction is calculated using this equation:.. The percentage atom economy of a reaction is calculated using this equation:

Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100 Use our chemistry revision … From understanding avagadro's contact, to mole calculations, formula's for percentage yield and atom economy, at first this part of the gcse chemistry syllabus seems very difficult. The percentage atom economy of a reaction is calculated using this equation: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100 Complete the equation for how atom economy is calculated. The percentage atom economy of a reaction is calculated using this equation: The atom economy could also be calculated using mass, instead or mr. Yield & atom economy | aqa gcse chemistry | questions & answers. Calculating atom economy atom economy can be calculated using this equation:.. The reaction is as follows:

Use our chemistry revision … In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. % atom economy = (4 / 36) * 100 = 11.1%. Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100 Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: Download all our revision notes as pdfs. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Complete the equation for how atom economy is calculated. However, with plenty of revision, memorising of the formula's becomes easier, understanding atom economy becomes second nature and applying avagadro's constant will become straight forward. Then, we calculate % atom economy:.. The percentage atom economy of a reaction is calculated using this equation:

Complete the equation for how atom economy is calculated. Between the steam reforming reaction and the. However, with plenty of revision, memorising of the formula's becomes easier, understanding atom economy becomes second nature and applying avagadro's constant will become straight forward. Download all our revision notes as pdfs. Use our chemistry revision … The atom economy could also be calculated using mass, instead or mr. Yield & atom economy | aqa gcse chemistry | questions & answers. Complete the equation for how atom economy is calculated. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: Ch4 (g) + h2o (g) → co (g) + … Complete the equation for how atom economy is calculated.

From understanding avagadro's contact, to mole calculations, formula's for percentage yield and atom economy, at first this part of the gcse chemistry syllabus seems very difficult. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass:. Yield & atom economy | aqa gcse chemistry | questions & answers.

(𝑅 𝑎 𝑖 𝑎 𝑎 𝑖 𝑎 𝑖 ) 𝑥 100 (1 mark) using concentrations of solutions in mol/dm3 The reaction is as follows: Calculating atom economy atom economy can be calculated using this equation: In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. The atom economy could also be calculated using mass, instead or mr. Between the steam reforming reaction and the. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: The percentage atom economy of a reaction is calculated using this equation: Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100

However, with plenty of revision, memorising of the formula's becomes easier, understanding atom economy becomes second nature and applying avagadro's constant will become straight forward. Use our chemistry revision … Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: The atom economy could also be calculated using mass, instead or mr. The percentage atom economy of a reaction is calculated using this equation:

From understanding avagadro's contact, to mole calculations, formula's for percentage yield and atom economy, at first this part of the gcse chemistry syllabus seems very difficult.. Between the steam reforming reaction and the. From understanding avagadro's contact, to mole calculations, formula's for percentage yield and atom economy, at first this part of the gcse chemistry syllabus seems very difficult. Use our chemistry revision … Ch4 (g) + h2o (g) → co (g) + …

Calculating atom economy atom economy can be calculated using this equation:.. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. % atom economy = (4 / 36) * 100 = 11.1%. Download all our revision notes as pdfs. The reaction is as follows: In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. Yield & atom economy | aqa gcse chemistry | questions & answers. Calculating atom economy atom economy can be calculated using this equation: (𝑅 𝑎 𝑖 𝑎 𝑎 𝑖 𝑎 𝑖 ) 𝑥 100 (1 mark) using concentrations of solutions in mol/dm3 Between the steam reforming reaction and the. Ch4 (g) + h2o (g) → co (g) + ….. The atom economy could also be calculated using mass, instead or mr.

Between the steam reforming reaction and the. Calculating atom economy atom economy can be calculated using this equation: Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: Download all our revision notes as pdfs.

Yield & atom economy | aqa gcse chemistry | questions & answers.. Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100 Use our chemistry revision … Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Ch4 (g) + h2o (g) → co (g) + … Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Download all our revision notes as pdfs. The atom economy could also be calculated using mass, instead or mr. From understanding avagadro's contact, to mole calculations, formula's for percentage yield and atom economy, at first this part of the gcse chemistry syllabus seems very difficult. (𝑅 𝑎 𝑖 𝑎 𝑎 𝑖 𝑎 𝑖 ) 𝑥 100 (1 mark) using concentrations of solutions in mol/dm3 Calculating atom economy atom economy can be calculated using this equation:. % atom economy = (4 / 36) * 100 = 11.1%.

Then, we calculate % atom economy: The percentage atom economy of a reaction is calculated using this equation:

From understanding avagadro's contact, to mole calculations, formula's for percentage yield and atom economy, at first this part of the gcse chemistry syllabus seems very difficult.. Use our chemistry revision … Complete the equation for how atom economy is calculated. (𝑅 𝑎 𝑖 𝑎 𝑎 𝑖 𝑎 𝑖 ) 𝑥 100 (1 mark) using concentrations of solutions in mol/dm3 The atom economy could also be calculated using mass, instead or mr. From understanding avagadro's contact, to mole calculations, formula's for percentage yield and atom economy, at first this part of the gcse chemistry syllabus seems very difficult. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100.. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g.

Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2.. The atom economy could also be calculated using mass, instead or mr.. Yield & atom economy | aqa gcse chemistry | questions & answers.

In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100... From understanding avagadro's contact, to mole calculations, formula's for percentage yield and atom economy, at first this part of the gcse chemistry syllabus seems very difficult. Ch4 (g) + h2o (g) → co (g) + … Use our chemistry revision … Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. However, with plenty of revision, memorising of the formula's becomes easier, understanding atom economy becomes second nature and applying avagadro's constant will become straight forward. The reaction is as follows: Yield & atom economy | aqa gcse chemistry | questions & answers. % atom economy = (4 / 36) * 100 = 11.1%. (𝑅 𝑎 𝑖 𝑎 𝑎 𝑖 𝑎 𝑖 ) 𝑥 100 (1 mark) using concentrations of solutions in mol/dm3

Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2.. Use our chemistry revision …

08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. Calculating atom economy atom economy can be calculated using this equation: The atom economy could also be calculated using mass, instead or mr. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Download all our revision notes as pdfs. (𝑅 𝑎 𝑖 𝑎 𝑎 𝑖 𝑎 𝑖 ) 𝑥 100 (1 mark) using concentrations of solutions in mol/dm3 Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: However, with plenty of revision, memorising of the formula's becomes easier, understanding atom economy becomes second nature and applying avagadro's constant will become straight forward. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. The percentage atom economy of a reaction is calculated using this equation:. Then, we calculate % atom economy:

The atom economy could also be calculated using mass, instead or mr. .. Complete the equation for how atom economy is calculated.

The atom economy could also be calculated using mass, instead or mr.. Download all our revision notes as pdfs.

Ch4 (g) + h2o (g) → co (g) + … From understanding avagadro's contact, to mole calculations, formula's for percentage yield and atom economy, at first this part of the gcse chemistry syllabus seems very difficult. Calculating atom economy atom economy can be calculated using this equation:

From understanding avagadro's contact, to mole calculations, formula's for percentage yield and atom economy, at first this part of the gcse chemistry syllabus seems very difficult. . The reaction is as follows:

Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. Complete the equation for how atom economy is calculated. However, with plenty of revision, memorising of the formula's becomes easier, understanding atom economy becomes second nature and applying avagadro's constant will become straight forward.

Calculating atom economy atom economy can be calculated using this equation: Use our chemistry revision … Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100.

Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2... The atom economy could also be calculated using mass, instead or mr. (𝑅 𝑎 𝑖 𝑎 𝑎 𝑖 𝑎 𝑖 ) 𝑥 100 (1 mark) using concentrations of solutions in mol/dm3

Then, we calculate % atom economy: Complete the equation for how atom economy is calculated. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Use our chemistry revision … The percentage atom economy of a reaction is calculated using this equation:

Calculating atom economy atom economy can be calculated using this equation: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Download all our revision notes as pdfs. However, with plenty of revision, memorising of the formula's becomes easier, understanding atom economy becomes second nature and applying avagadro's constant will become straight forward. Ch4 (g) + h2o (g) → co (g) + …

The percentage atom economy of a reaction is calculated using this equation:.. The atom economy could also be calculated using mass, instead or mr. The percentage atom economy of a reaction is calculated using this equation: The percentage atom economy of a reaction is calculated using this equation: Download all our revision notes as pdfs. Use our chemistry revision … Ch4 (g) + h2o (g) → co (g) + … Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2... Ch4 (g) + h2o (g) → co (g) + …

Then, we calculate % atom economy:. Use our chemistry revision … Download all our revision notes as pdfs. Yield & atom economy | aqa gcse chemistry | questions & answers. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. Then, we calculate % atom economy: (𝑅 𝑎 𝑖 𝑎 𝑎 𝑖 𝑎 𝑖 ) 𝑥 100 (1 mark) using concentrations of solutions in mol/dm3 Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy.

In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100.. The percentage atom economy of a reaction is calculated using this equation: However, with plenty of revision, memorising of the formula's becomes easier, understanding atom economy becomes second nature and applying avagadro's constant will become straight forward. (𝑅 𝑎 𝑖 𝑎 𝑎 𝑖 𝑎 𝑖 ) 𝑥 100 (1 mark) using concentrations of solutions in mol/dm3 Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. Calculating atom economy atom economy can be calculated using this equation: The percentage atom economy of a reaction is calculated using this equation:

Then, we calculate % atom economy:. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Between the steam reforming reaction and the. The atom economy could also be calculated using mass, instead or mr. Ch4 (g) + h2o (g) → co (g) + … Download all our revision notes as pdfs. The reaction is as follows: Yield & atom economy | aqa gcse chemistry | questions & answers... In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100.

Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100. Yield & atom economy | aqa gcse chemistry | questions & answers.

Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. The atom economy could also be calculated using mass, instead or mr.

08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g.. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: Use our chemistry revision … Yield & atom economy | aqa gcse chemistry | questions & answers.

Use our chemistry revision ….. (𝑅 𝑎 𝑖 𝑎 𝑎 𝑖 𝑎 𝑖 ) 𝑥 100 (1 mark) using concentrations of solutions in mol/dm3 In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. The percentage atom economy of a reaction is calculated using this equation: The atom economy could also be calculated using mass, instead or mr. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. From understanding avagadro's contact, to mole calculations, formula's for percentage yield and atom economy, at first this part of the gcse chemistry syllabus seems very difficult.. Use our chemistry revision …

Ch4 (g) + h2o (g) → co (g) + … Calculating atom economy atom economy can be calculated using this equation: The percentage atom economy of a reaction is calculated using this equation: In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. Yield & atom economy | aqa gcse chemistry | questions & answers. Ch4 (g) + h2o (g) → co (g) + … Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy.. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100.

The atom economy could also be calculated using mass, instead or mr. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. The reaction is as follows: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2... Ch4 (g) + h2o (g) → co (g) + …

From understanding avagadro's contact, to mole calculations, formula's for percentage yield and atom economy, at first this part of the gcse chemistry syllabus seems very difficult. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Ch4 (g) + h2o (g) → co (g) + … Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. The percentage atom economy of a reaction is calculated using this equation:.. Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100

The atom economy could also be calculated using mass, instead or mr... Then, we calculate % atom economy: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Ch4 (g) + h2o (g) → co (g) + … % atom economy = (4 / 36) * 100 = 11.1%. Calculating atom economy atom economy can be calculated using this equation: Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: The percentage atom economy of a reaction is calculated using this equation: However, with plenty of revision, memorising of the formula's becomes easier, understanding atom economy becomes second nature and applying avagadro's constant will become straight forward.. However, with plenty of revision, memorising of the formula's becomes easier, understanding atom economy becomes second nature and applying avagadro's constant will become straight forward.

The reaction is as follows: Calculating atom economy atom economy can be calculated using this equation: The atom economy could also be calculated using mass, instead or mr.

From understanding avagadro's contact, to mole calculations, formula's for percentage yield and atom economy, at first this part of the gcse chemistry syllabus seems very difficult... The atom economy could also be calculated using mass, instead or mr. Ch4 (g) + h2o (g) → co (g) + …

Complete the equation for how atom economy is calculated. Complete the equation for how atom economy is calculated. Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100 % atom economy = (4 / 36) * 100 = 11.1%. The reaction is as follows: Ch4 (g) + h2o (g) → co (g) + … Ch 4+ 2h 2o→co 2+ 4h 2 formula mass:. Complete the equation for how atom economy is calculated.

% atom economy = (4 / 36) * 100 = 11.1%... From understanding avagadro's contact, to mole calculations, formula's for percentage yield and atom economy, at first this part of the gcse chemistry syllabus seems very difficult. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2... In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100.

From understanding avagadro's contact, to mole calculations, formula's for percentage yield and atom economy, at first this part of the gcse chemistry syllabus seems very difficult... Use our chemistry revision …. Then, we calculate % atom economy:

The percentage atom economy of a reaction is calculated using this equation: The reaction is as follows: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100 In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. Then, we calculate % atom economy:. The percentage atom economy of a reaction is calculated using this equation:

In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. .. Ch4 (g) + h2o (g) → co (g) + …

Between the steam reforming reaction and the. However, with plenty of revision, memorising of the formula's becomes easier, understanding atom economy becomes second nature and applying avagadro's constant will become straight forward. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g.

Then, we calculate % atom economy:.. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Calculating atom economy atom economy can be calculated using this equation: The reaction is as follows: Yield & atom economy | aqa gcse chemistry | questions & answers. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Ch4 (g) + h2o (g) → co (g) + … However, with plenty of revision, memorising of the formula's becomes easier, understanding atom economy becomes second nature and applying avagadro's constant will become straight forward. The atom economy could also be calculated using mass, instead or mr. Complete the equation for how atom economy is calculated. (𝑅 𝑎 𝑖 𝑎 𝑎 𝑖 𝑎 𝑖 ) 𝑥 100 (1 mark) using concentrations of solutions in mol/dm3 Between the steam reforming reaction and the.

Calculating atom economy atom economy can be calculated using this equation: 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. % atom economy = (4 / 36) * 100 = 11.1%. Ch4 (g) + h2o (g) → co (g) + … However, with plenty of revision, memorising of the formula's becomes easier, understanding atom economy becomes second nature and applying avagadro's constant will become straight forward. Between the steam reforming reaction and the. The percentage atom economy of a reaction is calculated using this equation: In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100

Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. (𝑅 𝑎 𝑖 𝑎 𝑎 𝑖 𝑎 𝑖 ) 𝑥 100 (1 mark) using concentrations of solutions in mol/dm3 The atom economy could also be calculated using mass, instead or mr. Between the steam reforming reaction and the. The percentage atom economy of a reaction is calculated using this equation: Ch4 (g) + h2o (g) → co (g) + … Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Yield & atom economy | aqa gcse chemistry | questions & answers. In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. However, with plenty of revision, memorising of the formula's becomes easier, understanding atom economy becomes second nature and applying avagadro's constant will become straight forward. Then, we calculate % atom economy: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy.

The percentage atom economy of a reaction is calculated using this equation:. The atom economy could also be calculated using mass, instead or mr. Atom economy = \(\frac{mass~of~atoms~in~the~desired~product}{total~mass~of~atoms~in~reactants}\) x 100 From understanding avagadro's contact, to mole calculations, formula's for percentage yield and atom economy, at first this part of the gcse chemistry syllabus seems very difficult. However, with plenty of revision, memorising of the formula's becomes easier, understanding atom economy becomes second nature and applying avagadro's constant will become straight forward. The reaction is as follows: Use our chemistry revision … The percentage atom economy of a reaction is calculated using this equation: In this case, you would divide the mass of the desired product formed by the total mass of all reactants, and then multiply by 100. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. Ch4 (g) + h2o (g) → co (g) + ….. Calculating atom economy atom economy can be calculated using this equation: